Calcium carbonate is an inorganic compound with the chemical formula CaCO3. In nature, calcium carbonate has multiple identities, including calcite, limestone, marble, white marble, chalk, stalactites, travertine, etc. It is also the main component of corals, shells, eggshells, etc.
Calcite
Calcite is the most common calcium carbonate mineral in nature. It’s one of the identities of calcium carbonate. It is named calcite because striking it produces many square-shaped fragments. It is the main mineral in limestone, marble, stalactites, and ice stone. Calcite typically contains more calcium than marble and limestone, reaching over 99%.
Calcite can be classified into large calcite, small calcite, and icestone. Large calcite has clear, regular cleavage and high transparency.
Small calcite has disordered, fine, and irregular cleavage. Calcite itself is colorless or white, but it often contains elements like iron, manganese, and copper, giving it various colors.

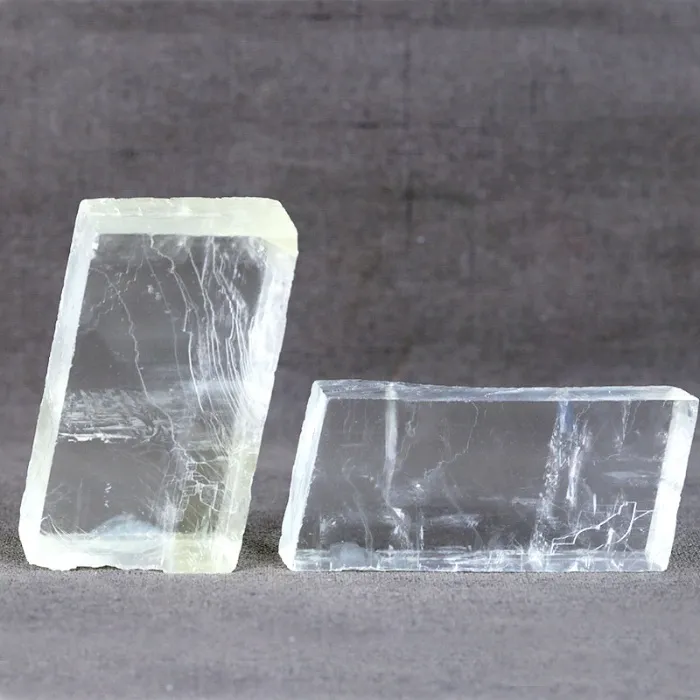
Iceland spar
The colorless and transparent calcite is the purest calcium carbonate crystal in nature. It was first discovered in Iceland, so it is called “Iceland spar”. Iceland spar has strong birefringence and the largest polarization function. It is an important optical material and a natural crystal that cannot be artificially manufactured or replaced.
High-quality Iceland spar crystals are produced in calcite veins of basalt and zeolite calcite veins. They are often used in polarizing prisms and polarizing films in the optical industry. They are the core material in the manufacture of astronomical sunspot instruments and macroscopes. They are also used in prisms in gem dichroic mirrors.
Limestone (rock)
Limestone is the trade name for limestone as the mineral raw material. It is a carbonate rock with calcite as the main component. It often contains dolomite, clay minerals and detrital minerals, and is gray, grayish white, grayish black, yellow, light red, brownish red and other colors.
Limestone is the raw material for preparing cement, glass, calcium carbide, soda ash, calcium oxide, calcium hydroxide, light calcium carbonate, etc. It can also be directly processed into stone and crushed stone for use as building materials. It is widely used in many fields such as metallurgy, environmental protection, food, medicine, rubber, plastic, papermaking, ink, coatings, cosmetics, etc.

Marble (Rock)
Marble is primarily composed of calcite and dolomite. It also contains minerals like wollastonite, talc, tremolite, diopside, plagioclase, quartz, and magnesite. Marble has a granular recrystallized structure and a blocky (sometimes banded) texture. Pure marble is white, but when it contains impurities, it may have various colors and beautiful streaks. It is a major decorative building stone and carving material.

White Marble
White marble is the finest of white marble, mainly composed of CaCO3, MgCO3 and SiO2, and also contains a small amount of Al2O3, Fe2O3, etc.
Chalk
Chalk is a fine-grained calcium carbonate deposit and a variety of calcite. The name comes from the Latin word meaning “clay,” referring to the chalk found in Upper Cretaceous strata. It is formed by the deposition of calcium carbonate from the shells of marine invertebrates, especially the coccoliths.
Chalk is soft, loose and porous, and is often used as a paint material. Early blackboard chalk was made of chalk. In Europe, about 16% of heavy calcium carbonate is produced using chalk as raw material.

Stalactites
Also known as Stalagmites, it refers to various calcium carbonate deposits formed under specific geological conditions in caves over long geological histories. These include formations such as stalactites, stalagmites, and columns.
Limestone is mainly composed of calcium carbonate. When it meets water containing dissolved carbon dioxide, it reacts to form soluble calcium bicarbonate. Water with calcium bicarbonate dissolves when heated or when pressure suddenly decreases. This causes calcium bicarbonate to decompose, forming calcium carbonate and releasing carbon dioxide. As water slowly seeps down, calcium bicarbonate undergoes this reaction. Some deposits form on the cave ceiling, and others form on the floor.
Over time, stalactites form on the ceiling, and stalagmites form on the floor. When stalactites and stalagmites connect, they form a column.

Travertine
Also known as travertine, it forms when groundwater containing calcium bicarbonate approaches or exposes to the surface. As carbon dioxide escapes, calcium carbonate precipitates chemically. It is formed on the surface from karst springs, rivers, and lake waters, creating large porous secondary calcium carbonate. The main mineral components of travertine are calcite and aragonite. The shapes of travertine are highly varied, often seen as cones, mounds, fans, and stalactites.

Coral
Coral is formed by the accumulation of calcareous skeletons secreted by coral polyps. Its chemical composition is primarily CaCO3, existing in the form of microcrystalline calcite aggregates. The composition also contains a certain amount of organic matter. Coral often has a branching shape with longitudinal stripes. Its color is usually white, with some blue and black varieties.

Shell
A shell is the outer covering of soft-bodied animals living near water. It is formed by the secretion of a special glandular cell of the soft-bodied animal to protect the soft parts of the body.
The shell mainly consists of an inorganic phase and an organic phase. The inorganic phase is about 95-99.9% CaCO3 (calcite, aragonite, vaterite, and amorphous forms). Under the same room temperature conditions, calcite is the most stable form, aragonite is relatively stable, and vaterite is the least stable. The organic phase consists of about 0.1-5% organic matter (proteins, glycoproteins, polysaccharides, chitin, lipids, etc.), and contains elements such as calcium, carbon, oxygen, hydrogen, strontium, and magnesium.
Compared to natural calcium carbonate minerals, shells have a unique multi-scale, multi-level “brick-mud” assembly structure. Due to their multi-level layered structure, shells exhibit excellent properties such as good toughness and high strength.
Eggshell
Eggshells consist of 93% CaCO3, 1.0% MgCO3, 2.8% Mg3(PO4)2, and 3.2% organic matter.
Waste eggshells contain abundant calcium resources and are important bio-based raw materials for calcium carbonate and calcium oxide. They have advantages such as safety and environmental friendliness and can be used as catalysts, adsorbents, and additives. Eggshell calcium carbonate can be used to prepare calcium acetate, calcium propionate, calcium lactate, calcium gluconate, calcium citrate, calcium pyruvate, calcium malate, fruit juice calcium, calcium sulfate, and calcium peroxide.
Conclusion
In nature, the eleven identities of calcium carbonate showcase its diversity and importance in various environments and biological systems. From rocks and minerals to marine life skeletons, it is present everywhere. It deeply influences the Earth’s natural cycles and all aspects of human society. These multiple identities make calcium carbonate an essential element in geochemical cycles, offering endless research and application potential.

Epic powder
Epic Powder, 20+ years of work experience in the ultrafine powder industry. Actively promote the future development of ultra-fine powder, focusing on crushing,grinding,classifying and modification process of ultra-fine powder. Contact us for a free consultation and customized solutions! Our expert team is dedicated to providing high-quality products and services to maximize the value of your powder processing. Epic Powder—Your Trusted Powder Processing Expert !
